Abstract
Introduction
Multiple myeloma (MM) is frequently accompanied by renal impairment (RI) in 30-40% patients at presentation (Dimopoulos et al, IMWG 2010). As per International Myeloma Working Group (IMWG), RI is defined as Creatinine clearance (CrCl) <40 mL/min. Patients with RI at diagnosis have inferior overall survival (OS), however, over the years their outcome has improved due to treatment with novel agents. We examined the baseline clinical characteristics, and outcomes in patients with a new diagnosis of multiple myeloma at our institution to identify factors associated with baseline RI and study prognostic trend over the last 2 decades.
Methods
We retrospectively reviewed records for MM patients with a new diagnosis of MM who were seen at Mayo Clinic, Rochester between 1/2004 and 12/2018. RI was defined as per IMWG criteria. Categorical variables were compared using chi-square test and continuous variables using Wilcoxon test. Overall Survival (OS) was estimated using Kaplan-Meier method and difference between groups was assessed using log-rank test.
Results
In our cohort of 2702 patients, median age at diagnosis was 65 (range, 22-96) years and 61% were males. Number of patients with CrCl <15, 15-29, 30-59 and ≥60 ml/min at diagnosis was 176 (6.5%), 204 (7.6%), 685 (25.3%) and 1637 (61%) respectively.
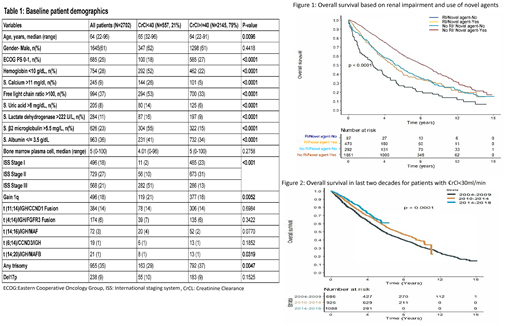
RI was present in 557 (21%) of patients at the time of presentation. On univariate analysis, factors significantly associated with RI at diagnosis were higher age, ECOG PS ≥2, hemoglobin< 10 g/dL, calcium >11 mg/dL , LDH >222 U/L, β-2-microglobulin >5.5 mg/L, free light chain ratio >100, uric acid >8 mg/dL, higher ISS stage and cytogenetics [gain 1q, t (14;20), absence of any trisomy] as shown in table 1. However, on multivariate analysis, only elevated serum calcium >11 g/dL (OR: 11.5; 95%CI:1.78-74.9; p=0.01) and free light chain ratio>100 (OR: 4.17; 95%CI:1.29-13.4; p=0.016) had significant association with RI.
The RI group had inferior OS when compared to patients with no RI at diagnosis (4.5 years vs 7.7 years, p-value <0.001). However, use of novel agents was associated with improvement in OS in patients with RI (4.9 years vs 2.6 years; p=0.0001) (Figure 1).
Looking at trends over time, the rate of RI at presentation had not changed. Over the last two decades, OS for the CrCl<30 group had significantly improved in the last 5-year period (3.8 years in 2004-2014 vs not reached for 2014-2018; p= 0.0064) as shown in figure 2.
Conclusion
In our cohort of 2702 patient, frequency of RI at baseline was 21%, which corroborates with previous studies. Hypercalcemia and serum free light chain ratio were found to be independent factors associated with RI. RI at diagnosis is associated with inferior survival, however, use of novel agents in this population has led to improvement of patient outcomes.
Kapoor: Karyopharm: Consultancy; Cellectar: Consultancy; BeiGene: Consultancy; Pharmacyclics: Consultancy; Sanofi: Consultancy; Amgen: Research Funding; Ichnos Sciences: Research Funding; Regeneron Pharmaceuticals: Research Funding; Glaxo SmithKline: Research Funding; Karyopharm: Research Funding; Sanofi: Research Funding; Takeda: Research Funding; AbbVie: Research Funding. Gertz: Aurora Biopharma: Other: Stock option; Ionis Pharmaceuticals: Other: Advisory Board; Akcea Therapeutics, Ambry Genetics, Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Karyopharm Therapeutics, Pfizer Inc (to Institution), Sanofi Genzyme: Honoraria; AbbVie Inc, Celgene Corporation: Other: Data Safetly & Monitoring; Akcea Therapeutics, Alnylam Pharmaceuticals Inc, Prothena: Consultancy. Dingli: Alexion: Consultancy; Novartis: Research Funding; Apellis: Consultancy; GSK: Consultancy; Sanofi: Consultancy; Janssen: Consultancy. Kumar: Roche-Genentech: Consultancy, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; Beigene: Consultancy; Novartis: Research Funding; Merck: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding; Bluebird Bio: Consultancy; Oncopeptides: Consultancy; Carsgen: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Tenebio: Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Antengene: Consultancy, Honoraria; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal